
Titanium Dioxide in Your Cosmetics: Decoding the Science on Safety
- Olivia Hart
- Skincare ingredients , Health science
- May 1, 2025
Table of Contents
Fast Facts: Titanium Dioxide in Cosmetics (TL;DR)
- Widely Used: Titanium dioxide (TiO₂) is a common mineral ingredient in sunscreens, makeup, and toothpaste for its UV filtering and pigment properties.
- Topical Safety: Scientific consensus is that TiO₂ is safe for topical use on intact skin, including nano-forms in sunscreens; it generally doesn’t penetrate the skin to reach the bloodstream.
- Food vs. Cosmetics: The EU banned TiO₂ (E171) as a food additive due to ingestion-related genotoxicity concerns for some nanoparticles. This ban does not directly apply to topical cosmetics, though oral cosmetic products (like toothpaste) are under scrutiny.
- Inhalation Concerns: Inhaling TiO₂ powder or spray is a concern, with IARC classifying TiO₂ dust as a Group 2B possible carcinogen. This is primarily an occupational hazard or risk with sprayable cosmetic products.
- Regulatory Status: TiO₂ is approved as a UV filter and colorant in cosmetics in the EU (with restrictions for inhalable products and ongoing assessment for oral cosmetics) and considered Generally Recognized as Safe and Effective (GRASE) for use in sunscreens by the US FDA.
- Benefit Outweighs Risk (Sunscreens): For sunscreens, the proven benefit of TiO₂ in preventing sun damage and skin cancer significantly outweighs hypothetical risks from topical application.
Titanium Dioxide (TiO₂, CI 77891) in Your Cosmetics: Should You Be Concerned? An Evidence-Based Look
Titanium dioxide (TiO₂), often listed as CI 77891 in ingredient lists, is a naturally occurring mineral extensively used in the cosmetics industry. You’ll find it in everything from sunscreens and foundations to powders and toothpastes. Its popularity stems from its brilliant white pigment and, crucially, its ability to effectively scatter and reflect harmful ultraviolet (UV) radiation, protecting our skin from sun damage, premature aging (photoaging), and skin cancer.
However, despite its widespread use and benefits, titanium dioxide has faced growing scrutiny from both scientific bodies and the public in recent years. Concerns primarily revolve around the safety of its nanoparticle forms and the potential for genotoxicity (damage to DNA). This article delves into the current scientific understanding, regulatory perspectives, and practical advice regarding TiO₂ in your cosmetic products.
What is Titanium Dioxide and Why Is It In Cosmetics?
Titanium dioxide is an inert, white mineral oxide. In cosmetics, it serves two main purposes:
- UV Filter: As a physical (or mineral) sunscreen, TiO₂ forms a barrier on the skin that blocks both UVA and UVB rays. It’s valued for its broad-spectrum protection and stability. It’s often used in conjunction with zinc oxide.
- Pigment: Its bright white color makes it an excellent opacifier and pigment, improving the texture and appearance of makeup products like foundations, powders, and eyeshadows, as well as whitening toothpastes.
TiO₂ can exist in different crystalline forms (mainly rutile and anatase) and particle sizes, including nanoparticles (typically defined as particles smaller than 100 nanometers). Nanoparticles are often preferred in sunscreens as they offer good UV protection without leaving a thick white cast on the skin.
The Core Safety Question: Nanoparticles, Ingestion, and Genotoxicity
The most significant recent development stirring the debate around TiO₂ safety was the European Food Safety Authority’s (EFSA) 2021 opinion on E171, the food additive grade of titanium dioxide. EFSA concluded that E171 could no longer be considered safe when used as a food additive. This decision was based on concerns that some TiO₂ nanoparticles, when ingested, could potentially accumulate in the body and cause DNA damage (genotoxicity), although the evidence was not definitive for all forms or conclusive about direct harm.
Crucially, this EFSA opinion and the subsequent EU ban on E171 in food products do not directly apply to cosmetic products applied topically. The exposure route (ingestion vs. skin application) is a key differentiator in risk assessment.
However, this has understandably raised questions about TiO₂ safety in cosmetics, especially for products that might be incidentally ingested (like lipsticks and toothpastes) or inhaled (like powders and sprays).
Regulatory Landscape: EU vs. US on Cosmetic TiO₂
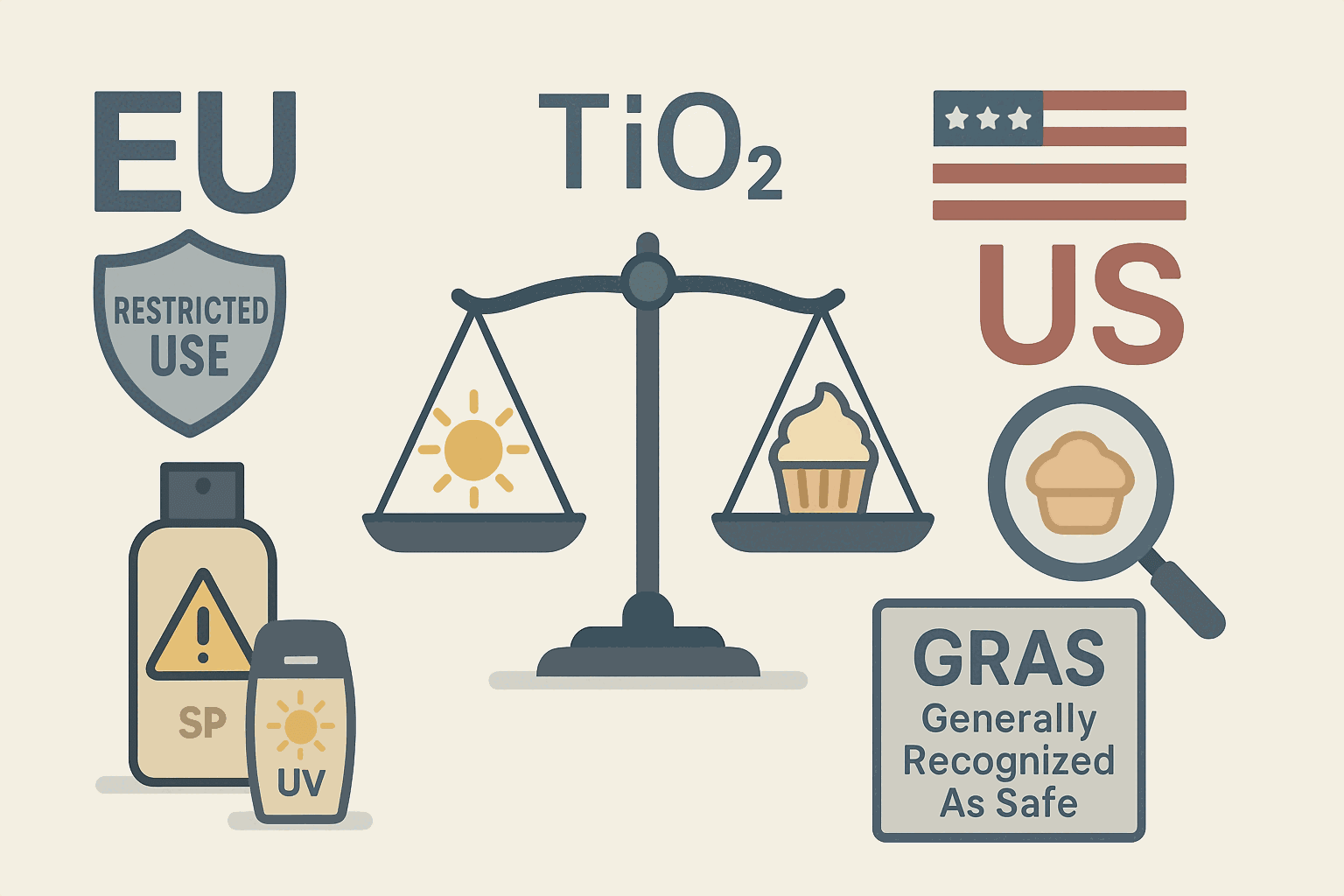 Regulatory bodies worldwide continuously evaluate the safety of cosmetic ingredients.
Regulatory bodies worldwide continuously evaluate the safety of cosmetic ingredients.
European Union (EU):
- The Scientific Committee on Consumer Safety (SCCS) is responsible for advising the European Commission on health risks of non-food consumer products.
- For topical use in sunscreens, the SCCS has repeatedly opined that titanium dioxide, including nano-forms (up to 25% concentration and under specific material characteristics), is safe when applied to healthy, intact, or sunburnt skin, as it does not appear to penetrate the deeper layers of the skin or enter the bloodstream in significant amounts.
- For inhalable products (e.g., sprays, powders), the SCCS has raised concerns. Their 2020 opinion (SCCS/1617/20) concluded that TiO₂ is not safe in certain concentrations in aerosol sprays due to potential lung inflammation upon inhalation. EU regulations restrict its use in applications that could lead to lung exposure by inhalation.
- For oral cosmetics (e.g., toothpastes, lip care), the SCCS has more recently stated that the genotoxicity of most TiO₂ grades used in such products cannot be excluded, mirroring some of EFSA’s concerns about ingestion. Further data is being sought.
United States (US):
- The Food and Drug Administration (FDA) regulates titanium dioxide.
- It is an FDA-approved color additive for use in cosmetics generally, including those for use around the eyes.
- As a sunscreen active ingredient, titanium dioxide is Generally Recognized As Safe and Effective (GRASE) for use in sunscreens up to a concentration of 25%.
Scientific Evidence: Breaking Down the Concerns for Topical Use
Let’s examine the key scientific points regarding TiO₂ in cosmetics applied to the skin:
1. Does TiO₂ Penetrate the Skin?
This is a central question, especially for nanoparticles. Numerous independent studies, including those reviewed by the SCCS and other bodies, have shown that titanium dioxide nanoparticles generally do not penetrate healthy, intact skin beyond the outermost layer (stratum corneum). They are not considered to reach viable skin cells or enter the bloodstream in significant quantities. Even with regular, long-term sunscreen use, systemic exposure via the skin is considered negligibly low. Some studies suggest a theoretical possibility of minor penetration through severely compromised skin or hair follicles, but this is not widely considered a significant health risk under normal use conditions.
2. Is TiO₂ a Carcinogen When Used Topically?
No, titanium dioxide is not classified as a carcinogen when used topically in cosmetics. The International Agency for Research on Cancer (IARC) has classified TiO₂ dust as “possibly carcinogenic to humans” (Group 2B), but this is based on studies involving high-level inhalation of powdered forms in industrial settings. This is primarily an occupational hazard for workers exposed to large amounts of airborne TiO₂ dust, not a risk associated with applying creams or lotions containing TiO₂ to the skin. The review in PubMed Central mentioned in the original draft suggested that historical inhalation risks might have been linked to contaminants rather than pure TiO₂ itself, though pure TiO₂ dust at high concentrations is still the focus of the IARC classification.
3. What About Other Potential Effects?
Beyond its primary role as a UV filter and pigment, some research suggests TiO₂ may possess antimicrobial properties and could potentially aid in wound healing. These effects are being explored for medical and therapeutic cosmetic applications but are not the primary reason for its use in everyday cosmetics.
Why Is TiO₂ Restricted or Scrutinized in Some Product Types?
The primary safety concerns that lead to restrictions or heightened scrutiny for TiO₂ are related to inhalation or ingestion of nanoparticles:
- Aerosol/Spray Products: Due to the risk of inhaling nanoparticles into the lungs, the EU has restrictions on TiO₂ in sprayable products.
- Loose Powders: Similar to sprays, loose powders can become airborne and inhaled, posing a theoretical risk, although consumer exposure levels are typically much lower than occupational ones.
- Toothpastes and Lip Products: These products carry a higher risk of accidental ingestion. The SCCS’s recent caution regarding the genotoxicity of ingested TiO₂ grades means these product categories are under careful review in the EU.
Should You Avoid Cosmetics Containing TiO₂?
Based on current scientific evidence collected over decades, there is no compelling reason to avoid topical cosmetic products containing titanium dioxide, especially sunscreens.
- Sunscreens: The effectiveness of sunscreens containing TiO₂ (and zinc oxide) in preventing sunburn, DNA damage from UV radiation, photoaging, and reducing the risk of skin cancer is extensively proven and widely accepted. The benefits of using sunscreen to protect against these known dangers far outweigh the largely hypothetical risks of topical TiO₂ application to intact skin. This is particularly true for individuals with fair skin or those with high sun exposure.
- Other Topical Cosmetics (creams, lotions, pressed makeup): For these products, where inhalation and ingestion risks are minimal, TiO₂ is generally considered safe.
If you are concerned:
- Choose non-nano options if preferred: Many brands offer sunscreens with non-nanoparticle TiO₂. Both forms are effective UV blockers.
- Be cautious with sprays and loose powders: If concerned about inhalation, opt for lotions or cream-based products instead of sprays or loose powders containing TiO₂.
- Toothpaste: Given the ongoing discussions about oral ingestion, you might choose a TiO₂-free toothpaste, especially for children who are more likely to swallow it. Many options are available.
Practical Takeaway: Don’t let unsubstantiated fears about titanium dioxide in sunscreen deter you from vital sun protection. The risk from UV radiation is significant and well-established. For other cosmetics, understanding the specific product type and exposure route is key.
What About Its Use in Food (E171)?
As mentioned, TiO₂ as a food additive (E171) was used to provide whiteness and opacity to products like chewing gum, candies, pastries, and sauces. The EU’s ban on E171 in food, effective from 2022, was a precautionary measure due to unresolved uncertainties about the genotoxicity of ingested nanoparticles, particularly regarding potential accumulation in the gut and effects on microbiota. The US and some other countries still permit its use in food within specified limits.
The key message remains: It’s prudent to avoid ingesting toothpaste containing TiO₂ (especially for children) and to be mindful of E171 if you are in a region where it’s still used in food and have concerns.
Conclusion: An Evidence-Based Perspective
Titanium dioxide remains a valuable and effective ingredient in topical cosmetic products, most notably in sunscreens where its photoprotective benefits are critical for skin health. Current scientific consensus, supported by major regulatory bodies like the FDA and the EU’s SCCS (for topical applications), indicates that TiO₂ is safe for use on the skin and does not pose a significant health risk when used as directed in non-inhalable, non-ingestible forms.
Concerns primarily arise from potential inhalation of airborne particles (relevant for sprays and loose powders, and occupational settings) and ingestion (relevant for the food additive E171 and oral care products like toothpaste). Research is ongoing, particularly for oral cosmetic products.
As a health-conscious consumer, it’s wise to stay informed. However, decisions should be guided by the weight of scientific evidence. For topical cosmetics, especially sunscreens, the protective benefits of TiO₂ are well-established and currently outweigh the largely unproven or route-specific risks.
Frequently Asked Questions (Q&A)
Q1: Is titanium dioxide (TiO₂) safe in sunscreen for daily use?
Q2: What are the main differences between titanium dioxide in food (E171) and in cosmetics?
Q3: Are nanoparticles of titanium dioxide in skincare products dangerous?
Q4: Why is titanium dioxide sometimes listed as a carcinogen?
Q5: Should I choose non-nano titanium dioxide sunscreen over nano?
Disclaimer
The information provided on BioBrain is intended for educational purposes only and is grounded in science, common sense, and evidence-based medicine. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare provider before making significant changes to your diet, exercise routine, or overall health plan.
References
- European Food Safety Authority (EFSA) (2021) "Safety assessment of titanium dioxide (E171) as a food additive"
- Scientific Committee on Consumer Safety (SCCS) (2014) "Opinion on Titanium dioxide (nano form) COLIPA S75. SCCS/1516/13. Revision of 22 April 2014"
- Gamer, A. O., Leibold, E., van Ravenzwaay, B. (2006) "The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin"
- Dréno, B., Alexis, A., Chuberre, B., Marinovich, M. (2019) "Safety of titanium dioxide nanoparticles in cosmetics"
- International Agency for Research on Cancer (IARC) (2010) "IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 93: Carbon Black, Titanium Dioxide, and Talc"
- U.S. Food and Drug Administration (FDA) (n.d.) "Titanium Dioxide (as a color additive)"
- U.S. Food and Drug Administration (FDA) (n.d.) "Sunscreen: How to Help Protect Your Skin from the Sun"
- CosmeticsInfo.org (n.d.) "Titanium Dioxide"
- Shi, H., Magaye, R., Castranova, V., Zhao, J. (2013) "Titanium dioxide nanoparticles: a review of current toxicological data"
- RIVM (2023) "Genotoxicity of titanium dioxide in cosmetics cannot be excluded"
- Scientific Committee on Consumer Safety (SCCS) (2020) "Opinion on Titanium dioxide (TiO2) used in cosmetic products that lead to exposure by inhalation. SCCS/1617/20"
- Campaign for Safe Cosmetics (n.d.) "Nanomaterials"
- Warheit, D. B. (2008) "Evaluating the health effects of inhaled nanomaterials: key lessons learned from the inhalation of pigment-grade titanium dioxide particles"
Tags :
- Titanium dioxide
- Ti o2 safety
- Cosmetic ingredients
- Nanoparticles in skincare
- Sunscreen safety
- E171
- Ci 77891
- Skin health
- Evidence based skincare